shelf life calculator for pharmaceutical products
ICH Q1E guideline provides guidance for the estimation of the shelf life of the pharmaceutical products and substances. Expiry with date in days in months or in years.

Estimate And 95 Confidence Limits Of Shelf Life Download Scientific Diagram
Overage It is over loading the dosage form with more drug than 100 ie 110 or more to give more time to get 90 potency ie.

. A pharmaceutical product is typically manufactured in batches. Shelf life is determined by the evaluation of whole stability data of the product. Accelerated aging studies can be used for shelf life determination but must later.
Long time studies on pharmaceutical products are carried out over extended time periods till the formulation fails its specifications under the recommended storage conditions. Establishing the Shelf Life of Pharmaceutical Products. The quality within each step influences the quality of the resulting knowledge.
Shelf life is longer. Batch and Product Shelf Life. Guidelines on Stability Studies of Pharmaceutical Products and Shelf Life Esti.
The shelf life of a product may vary between different countriesregions depending on regulatory requirements. ICH Q1E guideline provides guidance for the estimation of the shelf life of the pharmaceutical products and substances. A pharmaceutical product is typically manufactured in batches.
This can also be used to determine the shelf life of a product prior to purchase. Safeguarding and extending your products shelf life with perfect active packaging solution Preserving the freshness taste and texture of products till the time it. The labeled shelf life is what is printed on the drug products label and is used to calculate the expiry date.
Let ˆθbe an estimator of the true shelf-lifeθbased on y. Pacific Coast Composites Shelf Life Calculator is provided in order to help our customers determine the remaining shelf life of their product. QUANTILE REGRESSION CALIBRATION.
Therefore developing and instituting best scientific methods at each step supports the ongoing Quality-by. Why are expiration dates important for consumers to pay attention to. Minimum data of three batches are used to estimate the shelf life of pharmaceutical products.
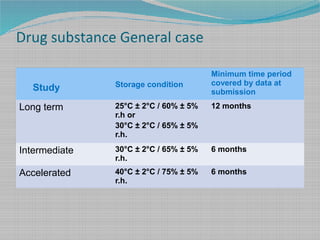
ASLT is a form Shelf-life assessment in which the stability or quality of the product is found out by keeping the sample in elevated conditions. Expiration Dates - Questions and Answers. The Q1A R2 guidelines offer test parameters and durations for long-term intermediate and accelerated studies that can be used for drug products or combination devices.
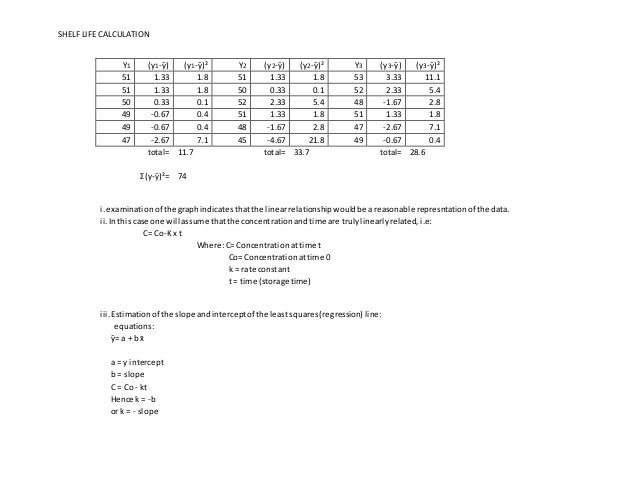
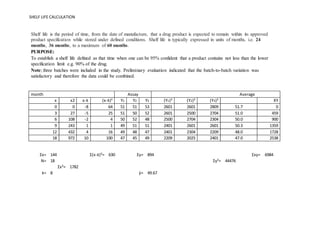
The variance estimateS²xyrepresentsthe variabilityof tabletpotencyatafixedtimeassumingitisequivalentacross all time points. Also it is necessary to find the mark. SHELF LIFE CALCULATION b N x ƩXY -ƩXx Ʃy -3024 N x ƩX² - ƩX² 11340 b -0267 mgmonth k 0267 a ӯ-b x x a 518 ivThe equationfora straightline bestfitis therefore.
1-Attached is a file describing the calculation method for half life. Thus the true shelf-life denoted byθ is the solution ofηαβx hence. As a result of the publication of 21 CFR Part 211 Current Good Manufacturing Practice for Finished Pharmaceuticals requirements were outlined concerning the expiration.
Batch and Product Shelf Life. At least three distinct steps are necessary to gain knowledge and understanding of a product or process. In order to calculate expiry date you should look at Production Date on your wrapping and write it into relevant field.
By this the deterioration rate is increased which effectively reduced the time of assessment. Accelerated stability testing and shelf-life calculation. The ASLT method was first introduced to the pharmaceutical product which was later adopted in food.
C Co - k x t C518 - 0267 x t v. This document assists with establishing the expiration period of a production bath of a medicinal productIt is not applicable to biological medicinal products such as vaccines sera toxins and allergens products derived from human blood and plasma as well as medicinal products prepared biotechnologically. This online service helps you to know how long your product is in good condition.
Pacific coast composites shelf life calculator is provided in order to help our customers determine the remaining shelf life of their product. Half life is t12 and shelf life is the time takes to degrade 10. θη αβNotethatαis the average drug characteristic at the time of manufacture iex 0 which is usually larger thanηThusθ0.
A batch is a fixed quantity of product for example 100000 tablets. The labeled shelf life is what is printed on the drug products label and is used to calculate the expiry date. The shelf life for a combination product is determined from drug stability device aging and sterile barrier aging with the shortest estimate determining the overall shelf life.
Stability Testing provides data that help assess a drug products stability and shelf life. This guideline applies to human and veterinary medicines. Drug expiration dates reflect the time period during which the product is known.
The expiry date for a batch of finished product shall be calculated from date of manufacture of product. Arrhenius plot to get k at 25 ºC and from this we can calculate the shelf-life. Pharmaceutical industry even after sitting on the shelf for a long time or when exposed to various.
A batch is a fixed quantity of product for example 100000 tablets. Typically storage is done at 250C - 20C and RH of 60 - 5 for up to 60 months. What should be the accelerated stability testing and shelf-life calculation is explained in the 21 CFR part 211137- Expiration dating.
For eg If the date of manufacture of a drug product is 5 Dec 2016 and the drug product has a shelf life of 2 years then the date of expiry shall be Nov 2018. The same equation can be used for calculating the shelf life. After entering data you push button Check and calculator will show you if.
Rational Shelf lives are usually a maximum of 5 years and it takes a product up to 2 years to reach customer Reduced shelf lives are seen in liquid products eg antibiotics and ophthalmics because they are unstable in. Enter all three dates to the left to see the remaining shelf life. Shelf life is a product of physical microbiological and chemical processes triggered by any one of a multitude of contributing factors.
Better estimates of product shelf life 378 months disincentive for industry to include more stability batches Pharmaceutical Stability Shelf Life August 1 2010 20 3-Batch Estimate of Shelf Life n 466 18 mean 229 months SD 586 Comparison of ICH Shelf Life Estimation Methodology Using Industry Data.

The Stability And Shelf Life Of Food

Evaluation Of Shelf Life Of Drug Products By Arrhenius Equation Part I Youtube
Shelf Life Calculation Of Drugs

Biopolimer Food Packaging Food6021 Binus Studocu

The Value Of Flexible Packaging In Extending Shelf Life And Reducing Food Waste Fpa Studies Conclude That Flexible Food Waste Reduce Food Waste Food Packaging

Shelf Life Estimation Methods Medicinal Chemistry

Shelf Life Of Foods First Order Kinetics Example Youtube

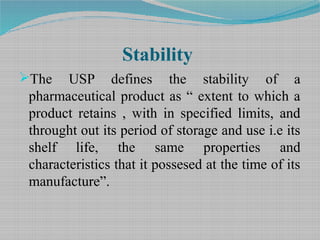
Stability Testing And Shelf Life Estimation

Wo2015122864a1 Accelerated Shelf Life Calculation Method Google Patents
Stability Testing And Shelf Life Estimation

Estimate And 95 Confidence Limits Of Shelf Life Download Scientific Diagram

Estimate And 95 Confidence Limits Of Shelf Life Download Scientific Diagram

Estimate And 95 Confidence Limits Of Shelf Life Download Scientific Diagram
Stability Testing And Shelf Life Estimation

Shelf Life Calculator For Composites And Other Materials
Shelf Life Calculation Of Drugs

Calculation Of Expiry Date Shelf Life Of Medicine By Accelerated Stability Study Method In English Youtube